
Our mission is to selectively modulate the cell’s molecular messenger, mRNA. Our name is a salute to another great messenger: Iris, the Greek goddess of communication.

Our mission is to selectively modulate the cell’s molecular messenger, mRNA. Our name is a salute to another great messenger: Iris, the Greek goddess of communication.
We have coined the term small binding RNA™, or sbRNA™, to represent our foundational technology. sbRNA™ constructs mimic natural RNAs and modulate gene expression through tightly regulated, highly selective binding events.
Each sbRNA™ is a short, ~20nt RNA sequence. No immunogenic protein expression required
We leverage a non-viral delivery modality that enables dosing to be increased, decreased, or stopped as needed
We achieve allele selectivity by directly targeting the expanded repeat sequence found among all patients with a given indication
One sbRNA™ can address multiple indications driven by the same repeat type
A single siRNA cuts the target

Multiple different small RNAs bind to the target mRNA

Multiple identical sbRNAs™ bind to the target mRNA
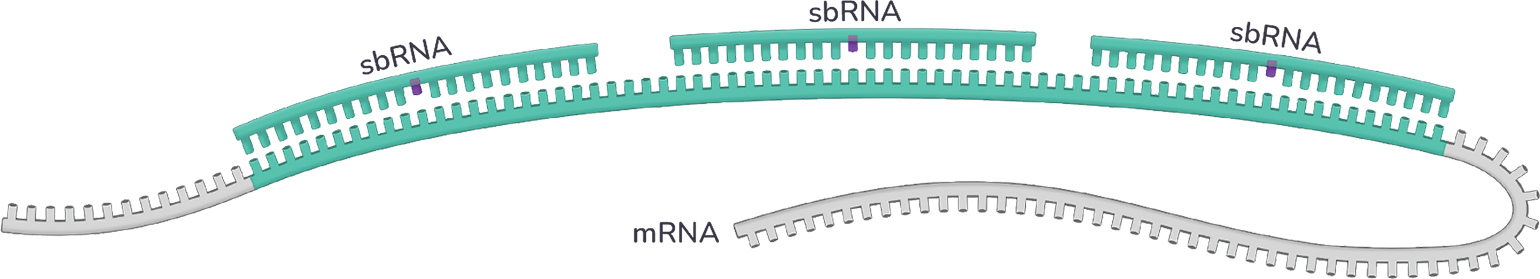
The sbRNA™ platform leverages the cooperativity inherent in the RNAi machinery to selectively modulate RNA transcripts harboring expanded, pathologic repeat sequences

A single sbRNA™ complex engages healthy transcripts, resulting in minimal to no inhibition. The result: Expression of that healthy gene is preserved.
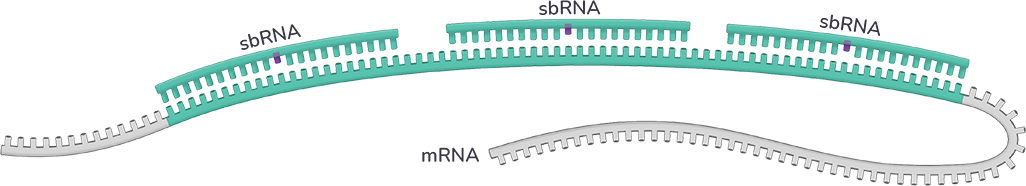
Multiple sbRNA™ complexes bind the expanded, mutant transcript simultaneously and in a cooperative manner, producing a high-affinity binding interaction. The result: Expression of the mutant gene is potently inhibited.
The repeat expansion disorders comprise a large family of over twenty devastating diseases with few to no treatment options today. The sbRNA™ technology directly targets the root cause of pathology in these diseases, the expanded repeat sequence. Furthermore, direct repeat targeting enables allele selectivity - for all patients. Our current lead programs are in Huntington’s Disease, C9orf72 ALS, and the Spinocerebellar Ataxias.




Camille (Cami) is a Partner at Venrock focused on healthcare with an emphasis on biotech, medical devices, and consumer health. Temperamentally an early-stage investor and company helper, Cami currently serves on the board of Cavalry, Iris, Mahzi, Ocelot, Unity (UBX), and is a board observer at Interdict. She previously served on the board of several exited Venrock portfolio companies, including Corvidia (sold to Novo), RegenXBio (RGNX), and Spirox (owned by Stryker). Prior to Venrock, Cami was a Managing Director at Versant Ventures where she served on the board (or as a board observer) at many companies including Genomic Health (GHDX/sold to Exact), Jazz (JAZZ), Kythera (KYTH/sold to Allergan), and Novacardia (sold to Merck). During her early career, Cami worked in corporate development at Genzyme, Millennium Predictive Medicine, and Tularik, and she was a management consultant at LEK.
Cami earned her Bachelor’s degree in Biology from Duke University and an MBA from Harvard Business School (HBS), where she graduated as a Baker Scholar. She is a Henry Crown Fellow, serves on the Investment Advisory Committee of Mission BioCapital (MBC), and serves on the HBS Global Advisory Board.
David (Dave) Maki has been associated with life science companies for the past 40 years. Prior to founding Altitude Life Science Ventures in 2004, Dave served as a partner with Perennial Ventures and previously as a vice president of Tredegar Investments, where he was responsible for the sourcing and management of investments in early-stage life science companies. From 1987 to 1999, Dave served as a partner at the intellectual property firm of Seed and Berry (now Seed IP Law), where he focused on strategic planning for healthcare companies, particularly in relation to R&D programs, competitive analysis, and corporate collaborations and licensing transactions. Under his leadership as senior partner in the biotechnology group, the firm built the client base to represent over 60 healthcare companies. During his career, Dave has served as a director for a diverse set of life sciences companies and has managed over 40 healthcare investments. Dave currently serves as a director on the board of Iris Medicine and Interdict Bio, and as an board observer at Tessera Therapeutics, Mozart Therapeutics, and Nutcracker Therapeutics, among others, and has previously served on the Board of Effector Therapeutics, Omniome and as Chairman of the Board of the Infectious Disease Research Institute.
Albert is the President and Chief Executive Officer of Homology Medicines and previously served as Chief Scientific Officer. Albert has spent more than 20 years coupling the discipline of human genetics with pharmaceutical R&D, resulting in the delivery of multiple therapeutic programs into development. At Homology, Albert is responsible for translating the Company’s in vivo AAVHSC platform into treatments for rare disorders. Through his leadership, Homology’s phenylketonuria (PKU) program advanced from scientific concept to the world’s first gene therapy clinical trial for PKU in just under three years, in addition to building a pipeline of genetic medicine programs in late-stage preclinical development. Prior to Homology, Albert was the Senior Vice President and Global Head of Research and Nonclinical Development at Shire, where he led a team responsible for the delivery of a sustained flow of rare disease therapeutics from idea to IND and supported the full R&D portfolio in the areas of toxicology, bioanalytics, drug metabolism and pharmacokinetics. Before joining Shire, he spent 14 years at Pfizer leading a team in the application of human genetics and computational biology to discover and develop therapeutics and pharmacogenomics strategies in diabetes, inflammatory diseases and oncology. He is a member of the Board of Directors of Ensoma. Albert received his undergraduate degree in Biology from the University of Delaware and M.S. degree in Molecular Biology from Johns Hopkins University. He received his Ph.D. and post-doctoral training in human genetics at the University of Pittsburgh.
Erica is Vice President & Head of UCB Ventures, an evergreen strategic corporate venture fund which was set up in 2017 to invest in innovative, early stage therapeutic opportunities beyond UCB’s current focus areas. She is on the Board of Directors of five other UCB Ventures portfolio companies: Rinri Therapeutics, ExeVir, Neurona, Splice Bio, and EsoBiotec. Erica began her career at UCB in 2010 initially in Strategy roles, and later as Head of Market Access and Pricing for EMEA Commercial Operations. Prior to joining UCB, Erica spent 10 years as a top-ranked biotechnology equity analyst for Merrill Lynch in London (1999-2009). Erica received BA degrees in Biology and in Comparative Literature from Brown University (Rhode Island USA). She obtained a PhD in Molecular Biology from the University of Edinburgh, and later an MBA from Heriot-Watt University Business School in Edinburgh. She enjoys running, cycling & family holidays.
Megan is the co-founder and President of Iris Medicine, where she has held positions of increasing responsibility overseeing corporate strategy, business development, and investor relations. Prior to Iris, Megan co-founded and served as Director of Business Development at Kelonia Therapeutics, a company pioneering tissue-specific gene delivery. Megan was previously a venture capital investor at Venrock with an emphasis on therapeutics and biotech company creation.
Megan earned a PhD in chemistry as a Hertz fellow with Benjamin Cravatt at The Scripps Research Institute. Her PhD work helped form the foundation of Vividion, which was acquired by Bayer in 2021. Megan received her AB/AM from Harvard, where she conducted research in the lab of Novel laureate EJ Corey. She has been named to the Forbes 30 under 30 list for Healthcare and Business Insider’s Under 40 in Biotech.
In addition to her work in biotech, Megan serves on the Board of two non-profits, Convergent Research and Pioneer Labs, that are dedicated to advancing early-stage research in areas of high unmet need.
David joined the UT Southwestern Pharmacology Department in 1992. He was promoted to Associate Professor with tenure in 1998 and Full Professor in 2003. In 2014, he was named the Rusty Kelley Professor of Medical Sciences. He is also a member of the Department of Biochemistry and the Simmons Cancer Center.
David is the President of the Oligonucleotide Therapeutics Society. He is also Executive Editor for Nucleic Acids Research (responsible for handling 250 manuscript per year) and is on the Editorial Board of Molecular Therapy Nucleic Acids, and Oligonucleotide Therapeutics. He is the author of more than 215 peer-reviewed papers and book chapters. He holds twelve approved patents. David has received funding from, among other sources, the Welch Foundation, NIH, the McKnight Award for Neuroscience Research, the American Heart Association, the Cure Huntington Disease Initiative, and Freidreich’s Ataxia Association. David has mentored dozens of technicians, graduate students, postdoctoral fellows, and visiting scientists.
Brigit is Adverum’s Chief Scientific Officer and is responsible for leading the company’s portfolio of drug discovery and development programs, including the AAV.7m8 platform. Brigit has more than 15 years of experience in the biotechnology industry and in academia, identifying and advancing promising drug candidates into the clinic. She has expertise across a broad range of technologies and disease areas, including gene therapy, small molecules, antibodies, protein degradation, central nervous system, and liver. Most recently, Brigit has been an advisor to several gene therapy companies. Previously, she was with Sangamo Therapeutics serving as Vice President, Discovery and Translational Research. She led the Hemophilia A program from initial gene cassette engineering to successful first-in-man clinical trial, resulting in a partnership with Pfizer. Additionally, Brigit led Sangamo’s central nervous system portfolio and pipeline expansion, ultimately leading to a partnership with Biogen. Brigit was also responsible for AAV engineering, liver rare disease, and discovery efforts in engineering antibodies.
Brigit earned her B.A. in Chemistry from Northwestern University, a Ph.D. in Biochemistry, Molecular Biology and Biophysics from University of Minnesota and was a postdoctoral scholar at Stanford University.
Fulvio leads Orchard Therapeutics’ discovery and translational research activities. He joined Orchard Therapeutics from Smart Immune where he served as Chief Scientific Officer. Previously, Fulvio was Senior Vice President of Translational Science at Audentes Therapeutics in San Francisco, where he was responsible for advancing the company’s pipeline from discovery to clinical development overseeing molecular biology, in vivo pharmacology, bioinformatics, and bioanalytics. Prior to joining Audentes, Fulvio was Chief Scientific Officer of Genethon in Évry, France from 2012 to 2017, where he led the development of a robust pipeline of gene therapy programs for blood, liver, and neuromuscular diseases. Earlier in his career, he held various positions at the Center for Regenerative Medicine of the University of Modena, Molmed SpA, Genera SpA, and the San Raffaele-Telethon Institute of Gene Therapy in Milan, Italy.
Fulvio is a member of the Scientific Advisory Board for Kriya Therapeutics. He is also a member of the European Molecular Biology Association, past member of the Board of the American Society of Gene and Cell Therapy, and a member of the editorial board of many international journals in the fields of genetics, molecular biology, and gene therapy. Fulvio earned a Ph.D. in medical genetics at the University of Rome School of Medicine and has published more than 200 articles in major international journals. He also serves as Professor of Molecular Biology at the University of Modena and Reggio Emilia in Modena, Italy.
John served as the founding Chief Executive Officer and a Director of Alnylam from 2002 to 2021, where he built and led the company from early platform research on RNA interference through global approval and commercialization of the first four RNAi therapeutic medicines, ONPATTRO®, GIVLAARI®, OXLUMO®, and Leqvio®. The fifth RNAi therapeutic, AMVUTTRA®, was approved in mid-2022. At Alnylam, he also led the company’s value creation strategy, building over $25B in market capitalization and forming over 20 major pharmaceutical alliances. He continues to serve on the Alnylam Scientific Advisory Board.
Prior to Alnylam, he was at Millennium Pharmaceuticals, Inc., where he was responsible for the company’s product franchises in oncology, and cardiovascular, inflammatory and metabolic diseases, in addition to leadership of M&A, strategy, and biotherapeutics functions. Before Millennium, he held scientific and business roles at Biogen, Inc. where he invented and led the discovery and development of ANGIOMAX® (bivalirudin) for injection. Previously, he was a scientist at ZymoGenetics, Inc. and the Upjohn Company.
John is currently a Venture Partner at ARCH Venture Partners, a Venture Advisor at Atlas Ventures, an Executive Partner at RTW Investments, a Senior Advisor for Blackstone Life Sciences, and an Advisor for M28. He is also member of the Board of Directors of publicly traded companies, including Agios Pharmaceuticals, Beam Therapeutics, Kymera Therapeutics, ProKidney Corp., and Takeda Pharmaceuticals. He is also on the Board of a number of private companies – including Aerium Therapeutics, Aera Therapeutics (Chair), Aitia (Chair), Hemab Therapeutics (Chair), Orbital Therapeutics (Executive Chair), and Versanis Bio. As the principal of JMM Innovation, LLC, John serves as a strategic advisor to a number of innovative biotechnology companies, including mentorship of CEOs in their mission to advance science and innovation for patients.
John is on the Board of the Biotechnology Innovation Organization, or “BIO,” where he was Chair from 2017-2019 and is Chair Emeritus. In addition, he serves on the Board of the Termeer Foundation – committed to continuing the legacy of the late Henri A. Termeer, as Chair of the n-Lorem Foundation Advisory Council – committed to meeting the needs of patients with nano-rare diseases, on the Advisory Board of Ariadne Labs – advancing global health system innovations, and as an advisor to Nucleate – a student-led organization facilitating the formation of pioneering life sciences companies.
John received his B.A., M.S. and Ph.D. in biochemistry and molecular biology at the University of Chicago.
Stuart is currently the CSO of Orfonyx Bio, responsible for all aspects of platform development and pipeline execution. In addition to his role at Orfonyx, he is a partner in Sunrise Bioventures, consults broadly across the nucleic acid therapeutics industry and is on a number of scientific advisory boards. Previously Stu was the Chief Platform Officer at Senda Biosciences (currently Sail Biomedicine), responsible for all aspects of the company’s novel nanoparticle platform and translational biology efforts. Prior to Senda, Stu was the VP of platform biology at Korro Bio, focused on RNA editing using ADAR. In this role he built and led the platform and screening teams and was part of the leadership team.
Stu got his start in biotech at Alnylam, where he spent nearly 12 years at the intersection of platform development and application. He was responsible for running the lead development team which helped to identify and optimize siRNAs for platform and therapeutic programs including all 5 currently approved siRNAs. Stu was also instrumental in initiating Alnylam’s effort to deliver siRNAs to the CNS and led a CNS platform and delivery group.
Stu received his PhD in Genetics from Stony Brook University/Cold Spring Harbor laboratory and did his postdoc in genomics at Dana-Farber Cancer institute with an appointment in the department of Genetics at HMS.

Iris Medicine is an early-stage life sciences company pioneering the future of RNA therapies. We are looking for highly creative and committed people to join our growing team.
To learn more, reach us at contact@irismedicine.com